
understand human embryogenesis and to develop regenerative
therapies for treating patients.
Like naive rodent PSCs, naive hPSCs can potentially be used to
generate interspecies chimeras for studying human development
and disease, and producing functional human tissues via interspe-
cies blastocyst complementation. To date, however, all reported
attempts on generating hPSC-derived interspecies chimeras
have used the mouse as the host animal, and the results obtained
suggest that this process is rather inefficient (Gafni et al., 2013;
Theunissen et al., 2014, 2016). Although the mouse is one of the
most important experimental models for stem cell research, there
are considerable differences between humans andmice (e.g., early
post-implantation development, embryo size, gestational length,
and developmental speed), which may hinder not only the effi-
ciency but also the usefulness of human-mouse chimeric studies.
Thus, expanding the repertoire of host species may complement
this incipient but promising area of research in the field of regener-
ative medicine. In particular, interspecies chimera research of
A
B
C
D
E
F
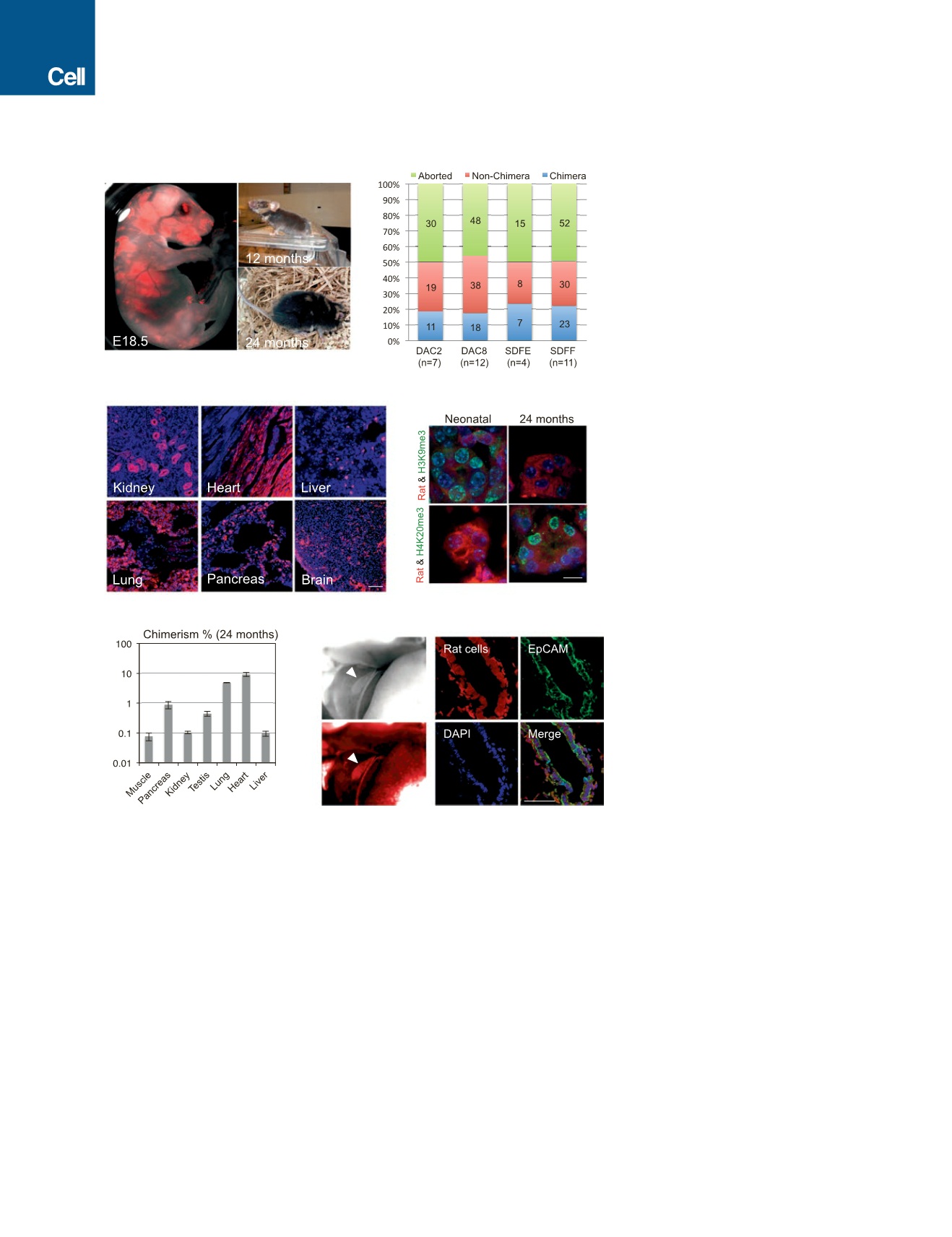
Figure 1. Interspecies Rat-Mouse Chimeras
Derived from Rat PSCs
(A) Rat-mouse chimeras generated by rat ESCs
(DAC2). Left, an E18.5 rat-mouse chimeric fetus. Red,
hKO-labeled rat cells. Right, a 12-month-old (top) and
24-month-old (bottom) rat-mouse chimera.
(B) Chimera forming efficiencies with rat ESC lines
(DAC2 and DAC8) and rat iPSC lines (SDFE and
SDFF). n, number of embryo transfers.
(C) Representative fluorescence images showing
hKO-labeled rat ESCs (DAC2) contributed to
different tissues in the 24-month-old rat-mouse
chimera. Red, hKO-labeled rat cells. Blue, DAPI.
Scale bar, 100
m
m.
(D) Representative immunofluorescence images
showing the expression of aging-related histone
marks, including H3K9me3 and H4K20me3, in the
kidney tissue of neonatal and 24-month-old chi-
meras. Scale bar, 10
m
m.
(E) Levels of chimerism of rat ESCs (DAC2) in
different tissues of the 24-month-old rat-mouse
chimera. Error bars indicate SD.
(F) Rat iPSCs (SDFE) contributed to the neonatal
mouse gall bladder. Left, bright-field (top) and
fluorescence (bottom) images showing a neonatal
mouse gallbladder contained cells derived from rat
iPSCs. White arrowheads indicate the gallbladder.
Right, representative immunofluorescence images
showing the expression of a gallbladder epithelium
marker (EpCAM) by rat cells. Red, hKO-labeled rat
cells; blue, DAPI. Scale bar, 50
m
m.
See also Figure S1 and Table S2.
hPSCs using ungulates, e.g., pigs, cattle,
and sheep, could lead to improved
research models, as well as novel in vivo
strategies for (1) generating human organs
and tissues, (2) designing new drug
screening methodologies, and (3) devel-
oping new human disease models (Wu
and Izpisua Belmonte, 2015). Experiments
to empirically test and evaluate the
chimeric contribution of various types of hPSCs in the ungulates
are thus imperative, but currently lacking. To start filling this void,
we tested different types of hPSCs for their chimeric contribution
potential in two ungulate species, pigs and cattle.
RESULTS
Naive Rat PSCs Robustly Contribute to Rat-Mouse
Interspecies Chimera Formation
We first used rodent models to gain a better understanding of the
factors and caveats underlying interspecies chimerism with
PSCs. To this end, we used two chimeric-competent rat ESC
lines, DAC2 and DAC8 (Li et al., 2008). We labeled both lines
with a fluorescent marker, humanized kusabira orange (hKO),
for cell tracking and injected them into mouse blastocysts.
Following embryo transfer (ET) into surrogate mouse mothers,
both DAC2 and DAC8 lines gave rise to live rat-mouse chimeras
(Figures 1A and S1A). Many of the chimeras developed into
474
Cell
168
, 473–486, January 26, 2017


















